1.B.49 The Anaplasma p44 (A-P44) Porin Family
Anaplasma phagocytophilum, an obligatory intracellular bacterium that causes human granulocytic anaplasmosis, has a small genome and needs to acquire many compounds from its host. The isolated outer membrane of A. phagocytophilum has porin activity as measured by a liposome swelling assay (Huang et al., 2007). The activity allows the diffusion of L-glutamine, the monosaccharides arabinose and glucose, the disaccharide sucrose, and even the tetrasaccharide stachyose, and this diffusion could be inhibited with an anti-P44 monoclonal antibody. P44s are the most abundant outer membrane proteins of A. phagocytophilum. The P44 protein demonstrates characteristics consistent with porins of gram-negative bacteria, including detergent solubility, heat modifiability, a predicted structure of amphipathic and antiparallel beta-strands, an abundance of polar residues, and a C-terminal phenylalanine. When reconstituted into proteoliposomes, purified P44s exhibited porin activity. P44s are encoded by approximately 100 p44 paralogs and go through extensive antigenic variation. The 16-transmembrane β-strands consist of conserved P44 N- and C-terminal regions. By looping out the hypervariable region, the porin structure is conserved among diverse P44 proteins yet enables antigenic variation for immunoevasion. The tricarboxylic acid (TCA) cycle of A. phagocytophilum is incomplete and requires the exogenous acquisition of L-glutamine or L-glutamate for function. Efficient diffusion of L-glutamine across the outer membrane suggests that the porin feeds the Anaplasma TCA cycle and that the relatively large pore size provides Anaplasma with the necessary metabolic intermediates from the host cytoplasm (Huang et al., 2007).
The transport reaction catalyzed by P44 is:
Small molecule (out) 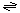 small molecule (in)
small molecule (in)