1.D.223. The Oxacalix[2]Arene[2]Triazine-derived Channel (OAT-C) Family
Anion transmembrane transport can be mediated by noncovalent molecular interactions. A series of oxacalix[2]arene[2]triazine-derived transporters (1 and 2) bearing anion-π-, hydrogen-, and halogen-bonding sites in rational proximity were designed and synthesized by a one-pot strategy starting from gallic acid ester derivatives and mono- or di-halogen-substituted triazines (Huang et al. 2019). 1H NMR titrations demonstrated efficient binding of 1 and 2 toward Cl- and Br- in solution, giving association constants in the range of 102-104 M-1. Cooperation of anion-π, hydrogen, and halogen bonding was revealed as a driving force for anion binding by single-crystal structures of two complexes and density functional theory calculations. Fluorescence assays indicated that compounds 1 are efficient chloride transporters with effective concentrations (EC50) falling in the range of 3.1-7.4 μM and following an order of 1a > 1b > 1c > 1d. The contribution of halogen bonding and cooperative noncovalent bonds to ion transport was discussed. Transporters 1 exhibit high anticancer activity. In the presence of 1 and KCl (60 mM), the cell survival of HCT116 reduces to 11.9-24.9% with IC50 values in the range of 52.3-66.4 μM.
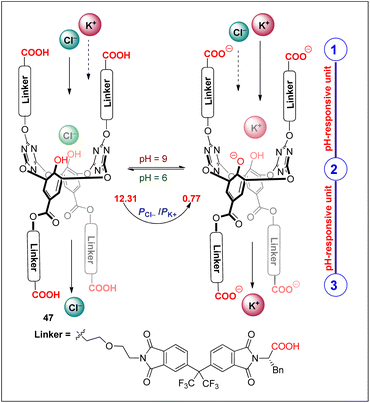
A monomolecular channel (47) mimics the natural Cl− channel (ClC) (Huang et al. 2020). 47 shows pH-dependent Cl− transport as well as selectivity (Fig. 31). At pH 6, the channel shows the highest Cl− transport with EC50 = 0.075 mol% and selectivity ratio PCl−/PK+ = 12.31. The selectivity decreases to 3.39 at pH 7 and 1.51 at pH 8. A further increase in pH to 9 results in a selectivity switch of PCl−/PK+ to 0.77. The rapid decrease in Cl− transport on increasing the pH is due to the deprotonation of the COOH groups (pKa ∼ 6) at the channel entrance, resulting in repulsion of Cl− ions. At pH 9, the phenolic OH (pKa = 8.21) of the central unit gets partially deprotonated and provides attractive forces for K+ transport (Kumar and Madhavan 2023).