| 1.C.5 The Channel-forming ε-toxin (ε-toxin) Family
The ε-toxin family consists of ε-toxin produced by Clostridium perfringens types B and D, which are responsible for a rapidly fatal enterotoxemia in sheep and other animals, as well as the mosquitocidal toxins, Mtx2 and Mtx3, produced by Bacillus sphaericus. These proteins are synthesized as relatively inactive prototoxins which are converted to active mature toxins by proteolytic removal of basic N-terminal peptides. ε-toxin increases intestinal and kidney cell permeability, forming a membrane complex of about 155 kDa which promotes efflux of intracellular K+ from target animal cells. The asymmetric pore complex (Nestorovich et al., 2010) is permeable to propidium ions and forms preferentially in the apical rather than the basolateral membrane (Petit et al., 2003). The mechanism of action of the mosquitocidal toxin is not known, but as these proteins are 20 to 27% identical to the ε-toxin of C. perfringens, they presumably function by a similar mechanism.
A 120-residue region of the ε-toxin of Clostridium perfringens (TC #1.C.5.1.1) shows significant sequence similarity to the pore-forming region of the pesticidal crystal protein Cry15Aa (insecticidal δ-endotoxin CryXVA(a)), and the first 77 residues of the epsilon toxin show sequence similarity with the first 82 residues of the beta-2 toxin of C. perfringens (TC #1.C.69). Cry15Aa is not demonstrably homologous to members of the channel-forming δ-endotoxin insecticidal crystal protein (ICP) family (TC #1.C.2). ε-toxin consists of a beta-barrel of 14 amphipatic beta strands (Popoff, 2011). The evidence presented by Knapp et al. (2009) suggests that the Aerolysin and RTX superfamilies may be distantly related.
ε-toxin (ETX) acts by heptamer formation and rapid
permeabilization of target cell membranes for monovalent ions with later entry of calcium. Knapp et al. (2009) compared the primary structure of ETX with that of the channel-forming
stretches of a variety of binding components of A-B-types of toxins
such as Anthrax protective antigen (PA), C2II of C2-toxin and Ib of
Iota-toxin and found homology to amino acids 151-180 of
ETX. Site-directed mutagenesis of amino acids within the putative
channel-forming domain resulted in changes of cytotoxicity and effects
on channel characteristics in lipid bilayer experiments including
changes in selectivity and partial channel block by
methanethiosulfonate (MTS) reagents and antibodies against His(6)-tags
from the trans-side of the lipid bilayer membranes.
The generalized transport reaction probably catalyzed by ε-toxin family members is:
Small solutes (in) 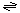 small solutes (out) small solutes (out)
|
This family belongs to the Aerolysin Superfamily.
|
| References: |
Brown, K.L., and H.R. Whiteley. (1992). Molecular characterization of two novel crystal protein genes from Bacillus thuringiensis subsp. thompsoni. J, Bacteriol. 174: 549-557.
|
Cases, M., J. Dorca-Arévalo, M. Blanch, S. Rodil, B. Terni, M. Martín-Satué, A. Llobet, J. Blasi, and C. Solsona. (2024). The epsilon toxin from Clostridium perfringens stimulates calcium-activated chloride channels, generating extracellular vesicles in Xenopus oocytes. Pharmacol Res Perspect 12: e70005.
|
Chan, S.W., T. Thanabalu, B.Y. Wee, and A.G. Porter. (1996). Unusual amino acid determinants of host range in the Mtx2 family of mosquitocidal toxins. J. Biol. Chem. 271: 14183-14187.
|
Cole, A.R., M. Gibert, M. Popoff, D.S. Moss, R.W. Titball, and A.K. Basak. (2004). Clostridium perfringens ε-toxin shows structural similarity to the pore-forming toxin aerolysin. Nat Struct Mol Biol 11: 797-798.
|
Freedman, J.C., B.A. McClane, and F.A. Uzal. (2016). New insights into Clostridium perfringens epsilon toxin activation and action on the brain during enterotoxemia. Anaerobe 41: 27-31.
|
Ji, B., J. Huang, K. Zou, M. Liu, Y. Pei, J. Huang, Y. Wang, J. Wang, R. Zhou, W. Xin, and J. Song. (2023). Direct Visualization of the Dynamic Process of Epsilon Toxin on Hemolysis. Small Methods e2300028. [Epub: Ahead of Print]
|
Knapp O., Maier E., Benz R., Geny B. and Popoff MR. (2009). Identification of the channel-forming domain of Clostridium perfringens Epsilon-toxin (ETX). Biochim Biophys Acta. 1788(12):2584-93.
|
Knapp, O., E. Maier, C. Piselli, R. Benz, C. Hoxha, and M.R. Popoff. (2020). Central residues of the amphipathic β-hairpin loop control the properties of Clostridium perfringens ε-toxin channel. Biochim. Biophys. Acta. Biomembr 1862: 183364. [Epub: Ahead of Print]
|
Liu, J.W., A.G. Porter, B.Y. Wee, and T. Thanabalu. (1996). New gene from nine Bacillus sphaericus strains encoding highly conserved 35.8-kilodalton mosquitocidal toxins. Appl. Environ. Microbiol. 62: 2174-2176.
|
Miyata, S., J. Minami, E. Tamai, O. Matsushita, S. Shimamoto, and A. Okabe. (2002). Clostridium perfringens ε-toxin forms a heptameric pore within the detergent-insoluble microdomains of Madin-Darby canine kidney cells and rat synaptosomes. J. Biol. Chem. 277: 39463-39468.
|
Nestorovich, E.M., V.A. Karginov, and S.M. Bezrukov. (2010). Polymer partitioning and ion selectivity suggest asymmetrical shape for the membrane pore formed by epsilon toxin. Biophys. J. 99: 782-789.
|
Okumura, S., H. Saitoh, N. Wasano, H. Katayama, K. Higuchi, E. Mizuki, and K. Inouye. (2006). Efficient solubilization, activation, and purification of recombinant Cry45Aa of Bacillus thuringiensis expressed as inclusion bodies in Escherichia coli. Protein Expr. Purif. 47: 144-151.
|
Petit, L., M. Gibert, D. Gillet, C. Laurent-Winter, P. Boquet, and M.R. Popoff. (1997). Clostridium perfringens ε-toxin acts on MDCK cells by forming a large membrane complex. J. Bacteriol. 179: 6480-6487.
|
Petit, L., M. Gilbert, A. Gourch, M. Bens, A. Vandewalle, and M.R. Popoff. (2003). Clostridium perfringens ε-toxin rapidly decreases membrane barrier permeability of polarized MDCK cells. Cell. Microbiol. 5: 155-164.
|
Popoff, M.R. (2011). Epsilon toxin: a fascinating pore-forming toxin. FEBS J. 278: 4602-4615.
|
Tilley, S.J. and H.R. Saibil. (2006). The mechanism of pore formation by bacterial toxins. Curr. Opin. Struct. Biol. 16: 230-236.
|
Wioland, L., J.L. Dupont, F. Doussau, S. Gaillard, F. Heid, P. Isope, S. Pauillac, M.R. Popoff, J.L. Bossu, and B. Poulain. (2015). Epsilon toxin from Clostridium perfringens acts on oligodendrocytes without forming pores, and causes demyelination. Cell Microbiol 17: 369-388.
|
| Examples: |
| TC# | Name | Organismal Type | Example |
| 1.C.5.1.1 | ε-toxin (epsilon toxin; ETx) type B precursor, EtxB, of 328 aas. Forms heptameric pores (Miyata et al. 2002). However, it has been reported to act on oligodendrocytes causing demyelination without forming pores (Wioland et al. 2015). The toxin acts on the brain, affecting vascular permeability, but also damaging neurons, astrocytes and oligodendrocytes (Freedman et al. 2016). The pore-forming regions are initially folded up on the surfaces of the soluble precursors. To create the transmembrane pores, these regions must extend and refold into membrane-inserted beta-barrels (Tilley and Saibil 2006). The crystal structure of epsilon-toxin revealed structural similarity to aerolysin from Aeromonas hydrophila.(Cole et al. 2004). Residues in the central position of each beta-strand of the amphipathic beta-hairpin loop that forms the transmembrane pore, control the size and ion selectivity of the channel (Knapp et al. 2020). The pre-pore morphology of ETX) has been provided (Ji et al. 2023). The ETX pore is formed in two stages: ETX monomers first attach to the membrane and form a pre-pore with no special conditions required, which then undergo a conformational change to form a transmembrane pore at temperatures above the critical point in the presence of receptors (Ji et al. 2023). Epsilon toxin stimulates calcium-activated chloride channels, generating
extracellular vesicles in Xenopus oocytes (Cases et al. 2024). | Firmicutes | EtxB or ETX of Clostridium perfringens |
| |
| 1.C.5.1.2 | Rhodanese-related sulfurtransferase of 319 aas and 1 N-terminal TMS. | | Sulfur transferase of Paenibacillus popilliae |
| |
| 1.C.5.1.3 | Poly-gamma-glutamate biosynthesis protein of 295 aas and 0 TMSs. | | PGG biosynthesis protein of Clostridium botulinum |
| |
| Examples: |
| TC# | Name | Organismal Type | Example |
| 1.C.5.2.1 | Mosquitocidal toxin, Mtx3 | Gram-positive bacteria | Mtx3 of Bacillus sphaericus (Q57028) |
| |
| 1.C.5.2.2 | Mosquitocidal toxin, Mtx2 | Gram-positive bacteria | Mtx2 of Bacillus sphaericus (Q45470) |
| |
| 1.C.5.2.3 | Uncharacterized protein of 319 aas, Sip1A | Firmicutes | Sip1A of Lysinibacillus sphaericus |
| |
| Examples: |
| TC# | Name | Organismal Type | Example |
| 1.C.5.3.1 | Parasporal crystal protein C53 | Gram-positive bacteria | C53 of Bacillus thurengiensis (Q45728) |
| |
 small solutes (out)
small solutes (out)