2.B.104. The Quinine-based Chloride Ion Transporter (Q-CIT) Family
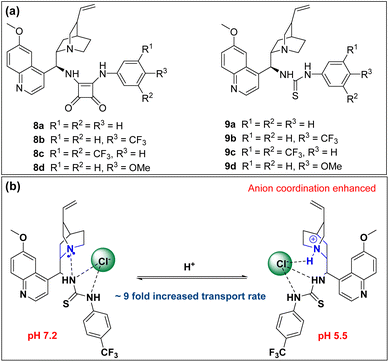
Quinine-based chloride ion transporters 8 and 9 have been reported by Akhtar et al. 2020. The design combines the conformational rigidity of quinines and the anion-binding capability of thiourea or squaramide units to form an anion-binding pocket. NMR titrations showed that the compounds have a higher affinity for chloride ions than for Br−, I− and NO3−. pH-dependent chloride transport was observed for all these compounds. Protonation of the quinidine N-atom at lower pH increased the extent of hydrogen bonding with anions, thereby increasing anion transport (see the figure below). The ratios of EC50 for pH 7.2/pH 5.5 for 8b and 9b were 5.3 and 8.9, respectively. This observation correlates with the corresponding pKa values of 8b (6.99) and 9b (6.96). The protonation of the nitrogen atom at low pH results in the enhancement of chloride transport. Mechanistic studies suggested H+/Cl− co-transport at pH 7.2.