2.B.63. The Diamidocarbazole Substituted Anion Transporter (DSAT) Family
Maslowska-Jarzyna et al. 2021 established 1,8-diamidocarbazoles as a versatile platform for the development of active chloride carriers. They investigated the influence of various electron-withdrawing substituents in positions 3 and 6 of the carbazole core on the chloride transport activity of these anionophores. Using a lucigenin assay and large unilamellar vesicles as models, the 3,6-dicyano- and 3,6-dinitro- substituted receptors were found to be highly active and perfectly deliverable chloride transporters, with EC50,270s value as low as 22 nM for Cl-/NO3 - exchange. Mechanistic studies revealed that diamidocarbazoles form 1:1 complexes with chloride in lipid bilayers and facilitate chloride/nitrate exchange by a carrier mechanism. Owing to its increased acidity, the 3,6-dinitro-substituted receptor acts as a pH-switchable transporter, with a physiologically relevant apparent pKa of 6.4. (Maslowska-Jarzyna et al. 2021).
Synthetic ionophores can also transport bicarbonate and chloride anions across lipid bilayers. Maslowska-Jarzyna et al. 2022 studied the bicarbonate and chloride transport by carbazoles with two amido/thioamido groups using a bicarbonate-sensitive europium(III) probe in liposomes and demonstrated a transporter concentration dependence. This was explained by a combination of two distinct transport mechanisms: HCO3-/Cl- exchange and a combination of unassisted CO2 diffusion and HCl transport, of which the respective contributions were quantified. The compounds studied were found to be highly potent HCl transporters. Based on mechanistic insights into anion transport, the authors tested the antimicrobial activity of these compounds and found a good correlation between their ion transport properties and their activity against Gram-positive bacteria (Maslowska-Jarzyna et al. 2022).
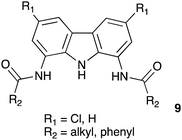
Structurally related 1,8-diamidocarbazoles have been studied as putative anion transporters by Chmielewski and co-workers. A family of compounds with general structure 9 (Figure below) was prepared, and their anion complexation, fluorescent sensing and anion transport properties across POPC membranes were studied. Various factors can affect the ability of compounds to transport anions including their lipophilicity, size and affinity for anionic guests. Derivatives with R1 = Cl and R2 = iso-butyl or tert-butyl were found to be the most active transporters in a chloride/nitrate exchange assay with EC50 values of 0.184 and 0.097 μM, respectively, demonstrating considerably more potent anion transport properties than the isophthalamides (TC Family 2.B.78). These compounds were also shown to be capable of facilitating chloride/bicarbonate antiport (Davis et al. 2020).