2.B.89. The 1,3-Bis(Benzimidazol-2-yl)Benzene Derivative (BBBD) Family
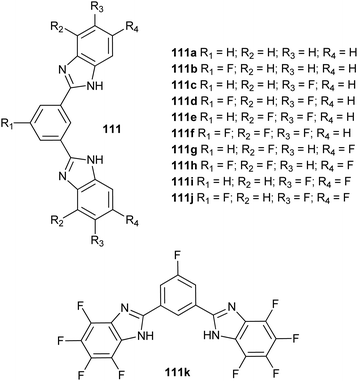
1,3-Bis(benzimidazol-2-yl)benzene derivatives 110 (see upper figure below) were explored by Yu et al. 2018. The anionophoric properties of these compounds were explored in EYPC liposomes using the HPTS and lucigenin assays. Derivatives bearing a nitro group in the central aromatic ring and benzimidazole moieties decorated with either –NO2 or –CF3 groups were found highly efficient with EC50 values down to 2.73 × 10−3% (36 nM) for 110c. The authors proposed these compounds to function as anion exchangers with contributions from proton/anion symport. Chloride transport mediated by these compounds in HeLa and MCF-7 cells was observed by treatment of these cells with the chloride-sensitive dye MQAE. Significant quenching of the fluorescence of the dye was observed upon treatment with the compounds, evidencing chloride internalisation mediated by these transporters. The cytotoxicity of these compounds was evaluated in several cancer cell lines. The most cytotoxic compound was found to be 110b. This toxicity diminished in a chloride-free buffer. These results evidence the role of anion transport in the toxicity exerted by these compounds. Hoechst 33342 and JC-1 staining suggested that these compounds induce apoptosis as a cell death mechanism. Yu et al. 2018 also explored the effect of fluorination in this type of compounds.96 Thus, fluorination of both the central aromatic unit and the benzimidazole moieties was performed to generate a small library of compounds 111 (see lower figure below). Fluorination was found to increase the transport activity of these compounds, with fluorination of the central aromatic unit being the most important factor to enhance this activity. The octa-fluorinated compound 111k was found to be the most efficient transporter, whereas the number and not the position of the fluorine atoms in the imidazole moieties was shown to be important. ISE assays determined that these compounds behaved as anion exchangers. Anionophoric activity in cells was evaluated using acridine orange and MQAE dyes to monitor acidic organelles basification and intracellular chloride increase, respectively. The cytotoxicity of these compounds was evaluated using the MTT assays in different cell lines. Again, this toxicity was amelioriated in the absence of chloride in the external buffer, and the calculated IC50 concentrations were in good agreement with the transport activities characterised for these derivatives, with 111k being the most cytotoxic. Disruption of the mitochondrial membrane potential was observed using JC-1 staining protocols. This result was evidence in support of apoptosis as the induced cell death mechanism (Davis et al. 2020).