2.B.7 The Transmembrane α-helical Peptide Phospholipid Translocation (TMP-PLT) Family
Kol et al. (2001; 2003) have demonstrated that membrane spanning segments of specific inner membrane proteins can catalyze translocation of phospholipids across the cytoplasmic membrane of E. coli. Transmembrane α-helical peptides corresponding to TMSs in known proteins mediate flip-flop of 2-6-(7-nitro-2,1,3-benzoxadiazol-4-yl) aminocaproyl (C6-NBD)-phospholipids. This has been demonstrated in proteoliposomes composed of E. coli phospholipids and a subset of bacterial membrane proteins. Leader peptidase of E. coli (225 aas; gi_16131214; 7 putative TMSs) and the KcsA potassium channel of Streptomyces lividans (TC #1.A.111) both induce phospholipid translocation although the E. coli ABC protein, MsbA (TC #3.A.1.106.1), and the E. coli OmpT outer membrane protease 3b (VII; gi_16128548) did not. These results suggest that some transmembrane α-helical segments can catalyze phospholipid translocation while others cannot.
The putative phospholipid transport reaction catalyzed by transmembrane α-helical peptides and proteins is:
phospholipid (inner monolayer of the bilayer) 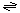 phospholipid (outer monolayer of the bilayer).
phospholipid (outer monolayer of the bilayer).