2.A.66 The Multidrug/Oligosaccharidyl-lipid/Polysaccharide (MOP) Flippase Superfamily
The MOP flippase superfamily includes eight distantly related families, five for which functional data are available: One ubiquitous family (MATE) specific for drugs, one (PST) specific for polysaccharides and/or their lipid-linked precursors in prokaryotes, one (OLF) specific for lipid-linked oligosaccharide precursors of glycoproteins in eukaryotes, one (AgnG) which includes a single functionally characterized member that extrudes the antibiotic, Agrocin 84, and one (MVI) of unknown transport function. The OLF family is found in the endoplasmic reticular membranes of eukaryotes. A broadly conserved Na+-binding site has been identified in the N-lobe of prokaryotic multidrug MATE transporters (Ficici et al. 2018).
All functionally characterized members of the MOP superfamily catalyze efflux of their substrates, presumably by cation antiport. Members of this family have been reported to have the MATE fold (Ferrada and Superti-Furga 2022). MATEs have been described as transporting primary and secondary metabolites, such as terpenoids, phenols, flavonoids, nicotine, alkaloids, phytohormones, proanthocyanidin, and anthocyanins (Saad et al. 2023).
2.A.66.1 The Multi Antimicrobial Extrusion (MATE) Family
The MATE family includes a functionally characterized multidrug efflux system from Vibrio parahaemolyticus NorM, and several homologues from other closely related bacteria that function by a drug:Na+ antiport mechanism, a putative ethionine resistance protein of Saccharomyces cerevisiae, a cationic drug efflux pump in A. thaliana and the functionally uncharacterized DNA damage-inducible protein F (DinF) of E. coli. The bacterial proteins are of about 450 amino acyl residues in length and exhibit 12 putative TMS. They arose by an internal gene duplication event from a primordial 6 TMS encoding genetic element. The yeast proteins are larger (up to about 700 residues) and exhibit about 12 TMSs. A conserved binding site in the N-lobe of prokaryotic MATE transporters suggests a role for Na+ in ion-coupled drug efflux (Castellano et al. 2021).
Human MATE1 (hMATE1) is an electroneutral H+/organic cation (OC) exchanger responsible for the final excretion step of structurally unrelated toxic organic cations in kidney and liver. Glu273, Glu278, Glu300 and Glu389 are conserved in the transmembrane regions. Substitution with alanine or aspartate reduced export of tetraethylammonium (TEA) and cimetidine, and several had altered substrate affinities (Matsumoto et al., 2008). Thus, all of these glutamate residues are involved in binding and/or transport of TEA and cimetidine, but their roles are different.
There are 59 MATE transporters in grapes (Vitis vinifera) (Watanabe et al. 2022). Group 1 may transport toxic compounds and alkaloids; Group 2 may transport polyphenolic compounds; Group 3 may transport organic acids, and Group 4 may transport plant hormones related to signal transduction. In addition to the known anthocyanin transporters, VvMATE37 and VvMATE39, a novel anthocyanin transporter, VvMATE38 in Group 2, was suggested as a key transporter for anthocyanin accumulation in grape berry skin. VvMATE46, VvMATE47, and VvMATE49 in Group 3 may contribute to Al3+ detoxification and Fe2+/Fe3+ translocation via organic acid transport (Watanabe et al. 2022).
The family includes hundreds of functionally uncharacterized but sequenced homologues from bacteria, archaea, and all eukaryotic kingdoms (Kuroda and Tsuchiya, 2009). A comprehensive review of the classes of efflux pump inhibitors from various sources, highlighting their structure-activity relationships, which can be useful for medicinal chemists in the pursuit of novel efflux pump inhibitors has appeared (Durães et al. 2018). A whole-body physiologically based pharmacokinetic study has characterized the interplay of OCTs (TC# 2.A.1.19) and MATEs in intestine, liver and kidney, predicting drug-drug interactions of metformin with perpetrators (Yang et al. 2021).
The probable transport reaction catalyzed by NorM, and possibly by other proteins of the MATE family is:
Antimicrobial (in) + nNa+ (out) → Antimicrobial (out) + nNa+ (in).
2.A.66.2 The Polysaccharide Transport (PST) Family
The protein members of the PST family are generally of 400-500 amino acyl residues in size and traverse the membrane as putative α-helical spanners twelve times. Analyses conducted in 1997 showed that they formed two major clusters. One is concerned with lipopolysaccharide O-antigen (undecaprenol pyrophosphate-linked O-antigen repeat unit) export (flipping from the cytoplasmic side to the periplasmic side of the inner membranes) in Gram-negative bacteria. On the periplasmic side, polymerization occurs catalyzed by Wzy. The other is concerned with exopolysaccharide or capsular polysaccharide export in both Gram-negative and Gram-positive bacteria. However, arachaeal and eukaryotic homologues are now recognized. The mechanism of energy coupling is not established, but homology with the MATE family suggests that they are secondary carriers. A review of Wzx undecaprenyl pyrophosphate (UndPP)-linked polysaccharide repeat units occurs by a substrate:product antiport mechanism (Islam and Lam 2012). These transporters may function together with auxiliary proteins that allow passage across just the cytoplasmic membrane or both membranes of the Gram-negative bacterial envelope. They may also regulate transport. Thus, each Gram-negative bacterial PST system specific for an exo- or capsular polysaccharide functions in conjunction with a cytoplasmic membrane-periplasmic auxiliary (MPA) protein with a cytoplasmic ATP-binding domain (MPA1-C; TC #3.C.3) as well as an outer membrane auxiliary protein (OMA; TC #3.C.5). Each Gram-positive bacterial PST system functions in conjunction with a homologous MPA1 + C pair of proteins equivalent to an MPA1-C proteins of Gram-negative bacteria. The C-domain has been shown to possess tyrosine protein kinase activity, so it may function in a regulatory capacity. The lipopolysaccharide exporters may function specifically in the translocation of the lipid-linked O-antigen side chain precursor from the inner leaflet of the cytoplasmic membrane to the outer leaflet (Islam and Lam 2012). In this respect they correlate in function with the members of the oligosaccharidyl-lipid flippase (OLF) family of the MOP flippase superfamily.
The generalized transport reaction catalyzed by PST family proteins is:
Polysaccharide (in) + energy → Polysaccharide (out).
2.A.66.3 The Oligosaccharidyl-lipid Flippase (OLF) Family
N-linked glycosylation in eukaryotic cells follows a conserved pathway in which a tetradecasaccharide substrate (Glc3Man9GlcNAc2) is initially assembled in the ER membrane as a dolichylpyrophosphate (Dol-PP)-linked intermediate before being transferred to an asparaginyl residue in a lumenal protein. An intermediate, Man5GlcNAc2-PP-Dol is made on the cytoplasmic side of the membrane and translocated across the membrane so that the oligosaccharide chain faces the ER lumen where biosynthesis continues to completion.
The flippase that catalyzes the translocation step is dependent on the Rft1 protein of S. cerevisiae (Helenius et al., 2002). Homologues are found in plants, animals and fungi including C. elegans, D. melanogaster, H. sapiens, A. thaliana, S. cerevisiae and S. pombe. The yeast protein, called the nuclear division Rft1 protein, is 574 aas with 12 putative TMSs. The homologue in A. thaliana is 401 aas in length with 8 or 9 putative TMSs while that in C. elegans is 522 aas long with 11 putative TMSs. These proteins are distantly related to MATE and PST family members and therefore are probably secondary carriers.
2.A.66.4 The Mouse Virulence Factor (MVF) Family
A single member of the MVF family, MviN of Salmonella typhimurium, has been shown to be an important virulence factor for this organism when infecting the mouse (Kutsukake et al., 1994). In several bacteria, mviN genes occur in operons including glnD genes that encode the uridylyl transferase that participates in the regulation of nitrogen metabolism (Rudnick et al., 2001). Nothing more is known about the function of this protein or any other member of the MVF family. However, these proteins are related to members of the PST and MATE families (>9 S.D.), and the greatest sequence similarity is found with members of the PST family. It is therefore possible that MVF family members are functionally related to PST family members and catalyze efflux by a cation antiport mechanism.
2.A.66.5 The Agrocin 84 Antibiotic Exporter (AgnG) Family
Agrocin 84 is a disubstituted adenine nucleotide antibiotic made by and specific for Agrobacteria. It is encoded by the pAgK84 plasmid of A. tumefaciens (Kim et al., 2006) and targets a tRNA synthetase (Reader et al., 2005). The agnG gene encodes a protein of 496 aas with 12-13 putative TMSs and a short hydrophilic N-terminal domain of 80 residues. AgnG is distantly related to members of the Mop superfamily, but is so distant, that it does not retrieve any such members in a TC BLAST search. Nevertheless, an NCBI BLAST search retrieves proteins of the PST and MVI families without iterations. agnG null mutants accumulate agrocin 84 intracellularly and do not export it (Kim et al., 2006).
The reaction catalyzed by AgnG is:
agrocin (in) 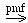 agrocin (out)
agrocin (out)
2.A.66.6 The Putative Exopolysaccharide Exporter (EPS-E) Family
2.A.66.7 Putative O-Unit Flippase (OUF) Family
2.A.66.8 Unknown MOP-1 (U-MOP1) Family
2.A.66.9 The Progressive Ankylosis (Ank) Family
Craniometaphyseal dysplasia (CMD) is a bone dysplasia characterized by overgrowth and sclerosis of the craniofacial bones and abnormal modeling of the metaphyses of the tubular bones. Hyperostosis and sclerosis of the skull may lead to cranial nerve compressions resulting in hearing loss and facial palsy. An autosomal dominant form of the disorder has been linked to chromosome 5p15.2-p14.1 within a region harboring the human homolog (ANKH) of the mouse progressive ankylosis (ank) gene. The ANK protein spans the cell membrane and shuttles inorganic pyrophosphate (PPi), a major inhibitor of physiologic and pathologic calcification, bone mineralization and bone resorption (Nurnberg et al., 2001).
The ANK protein has 12 membrane-spanning helices with a central channel permitting the passage of PPi. Mutations occur at highly conserved amino acid residues presumed to be located in the cytosolic portion of the protein. The PPi channel ANK is concerned with bone formation and remodeling (Nurnberg et al., 2001).
2.A.66.10 LPS Precursor Flippase (LPS-F) Family
2.A.66.11 Uncharacterized MOP-11 (U-MOP11) Family
2.A.66.12 Uncharacterized MOP-12 (U-MOP12) Family