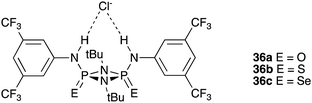
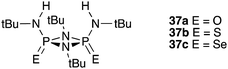
2.B.86. The Phosphazane Anion Carrier (PAC) Family
Plajer et al. 2020 explored the anion binding and transport properties of a wide range of compounds (including the selenium analogue 36c) (see the top figure below) and also compounds 37a–c (see the second figure below). These researchers determined the stability constant of 36c with chloride by UV/vis titration techniques in acetonitrile. They found that this compound binds chloride with a log K of 5.74 ± 0.05. For phosphazanes 37a–c, 1H NMR titration techniques were used in acetonitrile-d3. In this case the stability constants were found to be 37c (235 ± 7 M−1) > 37b (155 ± 3 M−1) > 37a (18 ± 2 M−1).
The anion transport properties of these compounds were studied using
both lucigenin (to study chloride transport and anion exchange) and
HPTS based assays (to study selectivity) in POPC LUVs. The results of
the lucigenin assay with 36c and 37c showed that compound 36c is the better anion transporter of the two systems. This was
rationalized by its higher affinity for chloride and the presence of the
CF3 functionalized aromatic groups. The HPTS assay showed
that the compounds function as anion antiport agents. The anion
transport properties of phosphazane 36c were compared to organic analogues containing 3,5-bis(trifluoromethyl) phenyl groups from a previous study with 36c EC50 = 8.56 nM as compared to the analogous thiourea EC50 = 8.02 nM and squaramide EC50 = 0.35 nM. The researchers also compared the anion transport ability of the series 37a–c and found an increase in membrane transport activity on moving to the heavier chalcogens (i.e., transport activity followed the trend 37c > 37b > 37a).
The authors attributed this to both the affinity of the transporters
for chloride (Se > S > O) and the matching trend in their
hydrophobicity. Plajer et al. 2020 have also introduced
metal-coordinating groups into this scaffold to pre-organize the anion
binding site and enhance anion transport activity.