1.B.27 The Helicobacter Outer Membrane Porin (Hop) Family
The Hop family consists of 31 outer membrane proteins in Helicobacter pylori. Five such proteins, HopA-HopE have been shown to function as porins both in bacterial outer membranes and in reconstituted planar bilayer membranes. The HopE protein appears to form a β-barrel with 16 transmembrane amphipathic β-strands. It is the smallest of the characterized Hop family members, but it forms the largest channels with a single channel conductance of 1.5 nS in 1M KCl (Bina et al. 2000).
Helicobacter pylori attaches to gastric tissue through members of a paralogous family of 'Helicobacter outer membrane proteins' (Hops), including adhesins BabA, SabA, HopQ, LabA and HopZ. Hops share a conserved 25 kDa C-terminal region that is thought to form an autotransporter-like transmembrane domain. Hops contain a non-continuous transmembrane domain, composed of seven predicted beta-strands at the C-terminus and one at the N-terminus (Coppens et al. 2018). Folding and outer membrane localization of the C-terminal beta-domain depends on a predicted transmembrane beta-strand within the first 16 N-terminal residues. The N-terminus resides in the periplasm, and the crystal and small angle X-ray scattering structures for the SabA extracellular domain reveal a conserved coiled-coil stem domain that connects to transmembrane beta-strands 1 and 2. Thus, Hop adhesins represent a novel outer membrane protein topology encompassing an OmpA-like 8-stranded beta-barrel that is interrupted by a 15 - 108 kDa domain inserted inside the first extracellular loop. The insertion of large, folded domains in extracellular loops is unprecedented in bacterial outer membrane proteins (Coppens et al. 2018). It is possible that these proteins are autotransporters.
The generalized transport reaction catalyzed by Hop family porins is:
solute (out) 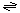 solute (periplasm)
solute (periplasm)